Recent News
Not your mother’s nanomedicine: NIH grant targets safer medication for women
October 20, 2025
AI tensor network-based computational framework cracks a 100-year-old physics challenge
September 16, 2025
Postdoctoral research fellow wins 1st place in conference poster presentation
August 25, 2025
Engineering a new treatment for ovarian cancer
June 24, 2025
News Archives
Professor Datye's Research Recognized in the Top Ten of C&EN
December 15, 2016
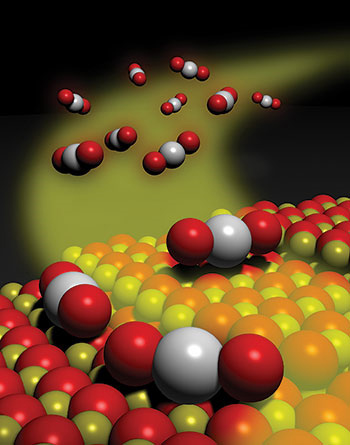
Chemical & Engineering News (C&EN) recently announced their notable chemistry research advances for 2016. Professor Abhaya K. Datye's research on thermally stable single-atom platinum-on-ceria catalysts via atom trapping (published in Science 8 July 2016) made the top 10.
From C&EN: Mediating chemical reactions with a single catalytically active atom on a solid surface has seemed like a good idea, albeit a fanciful one, for some time. This singular approach to catalysis gained traction this year as researchers demonstrated that such reactions are indeed feasible.
Tiny particles of a catalytic material—often a noble metal such as platinum—dispersed on a metal oxide or other solid support material are workhorses for industrial-scale chemical processes, including converting crude oil to valuable products such as gasoline. Compared with these conventional multiatom catalysts, single-atom versions would greatly reduce the consumption of costly and scarce precious metals. In addition, the atomic-scale uniformity would minimize unwanted reactions and by-products and simplify researchers’ efforts to deduce reaction mechanisms, a key step in making better catalysts.
In one example this year, Abhaya K. Datye of the University of New Mexico and coworkers showed that exposing platinum nanoparticles to hot oxidizing conditions causes the metal to form volatile PtO2, which desorbs from the nanoparticles. The researchers trapped that mobile supply of platinum atoms on a nearby cerium oxide surface, forming a catalyst for CO oxidation, a key reaction in engine emission cleanup (Science 8 July 2016).
